MXenes in Drug Delivery
Breast cancer is the most common type of cancer in women and one of the main causes of cancer-related deaths around the world. It affects women of all ages, ethnicities, and backgrounds, making it a major public health concern. Even though there have been big advances in cancer treatments such as targeted medicines, immune therapies, and nanomedicines, breast cancer is still difficult to treat effectively. This is mainly because cancer cells can become resistant to drugs, tumors vary from person to person, and medicines often do not reach the tumor site in sufficient amounts.
Nanomedicine, using nanoparticles to deliver drugs, has shown great promise for detecting, treating, and monitoring breast cancer. Different types of nanoparticles, such as metal-based, polymer-based, and two-dimensional (2D) nanomaterials, have been developed (1). However, these nanomedicines still face problems like poor compatibility with the body, uneven distribution, unwanted side effects, and drugs being released too early or in the wrong place (2). The complex structure of tumors also makes it hard for nanoparticles to reach all parts of the cancer, reducing their effectiveness (3). Therefore, new and smarter nanodelivery systems are needed to make treatments safer, more precise, and more effective.
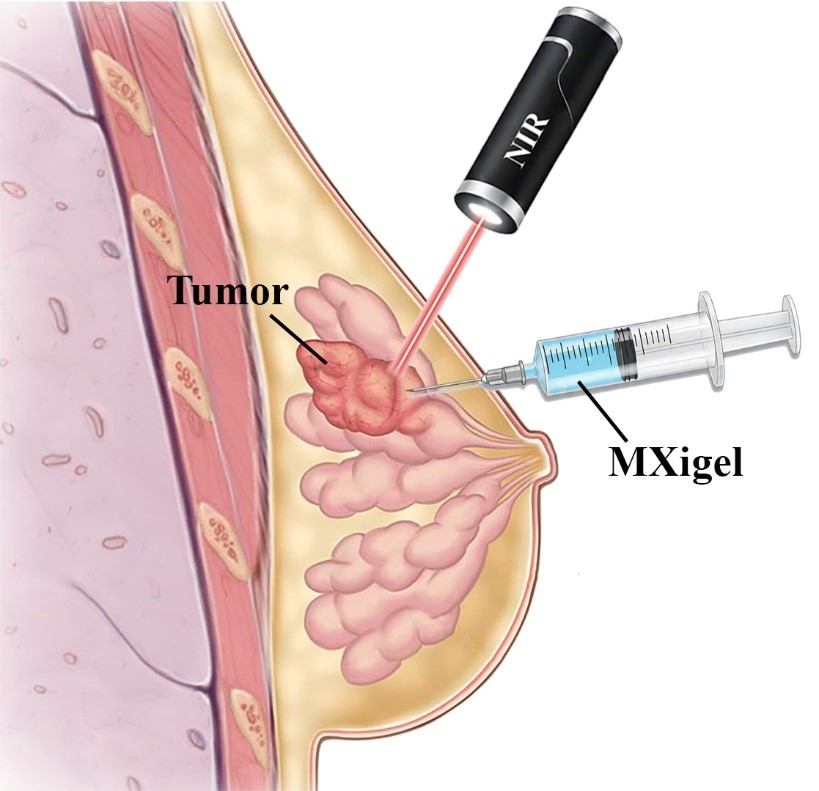
One promising approach is localized nanodelivery, which means delivering the medicine directly into the tumor instead of throughout the whole body. This method increases the amount of drug at the cancer site while reducing harmful side effects elsewhere (4). Hydrogels are especially useful for this purpose. They can slowly release drugs, stay at the tumor site for longer, and interact well with the body’s tissues (5).
Recently, scientists attracted to combining MXenes, a special type of 2D material transition metal carbides and nitrides, with hydrogels. MXenes have excellent heat and electrical properties and can be easily modified for medical use (6). When mixed with hydrogels, they create MXene-based hydrogels, which can both deliver drugs and generate heat when exposed to near-infrared (NIR) light. This allows for dual-action therapy, chemotherapy to kill cancer cells and photothermal therapy (PTT), where controlled heat destroys tumor tissue.
In this project, we aim to develop a smart MXene-based hydrogel system for breast cancer treatment. This system will deliver chemotherapy drugs directly to the tumor and use light to trigger additional heat-based therapy. The goal is to make treatment more effective, reduce side effects, and create a next-generation, intelligent platform for localized cancer therapy.
References
- Ashkarran, A., et al. Impact of nanomedicine in women's metastatic breast cancer. Small, (2024): 20(41), 2301385.
- Rosenblum, D., et al. Progress and challenges towards targeted delivery of cancer therapeutics. Nature Communications, (2018): 9(1), 1410.
- Li, J., et al. Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharmaceutica Sinica B, (2020): 10(11), 2110-2124.
- Shaha, S., et al. Locoregional drug delivery for cancer therapy: Preclinical progress and clinical translation. Journal of Controlled Release, (2024): 367, 737-767.
- Haider, A., et al. Advances in chitosan-based drug delivery systems: A comprehensive review for therapeutic applications. European Polymer Journal, (2024): 210, 112983.
- Güngör, A., et al. Electrochemical and Defect Characterization of APTES‐Functionalized Ti3C2Tx MXene for Supercapacitor Devices. (2024): Small: e05698.
Author: Mina Masoudi, PhD
NanoBio4Can MSCA Co-Fund Fellow
Sabancı University Nanotechnology Research and Application Center (SUNUM)