Combining Two Treatments in One Smart Nanosystem
As a NanoBio4Can fellow, my research focuses on developing lipid nanoparticles (LNPs) as advanced carriers for combination drug delivery. These nanoparticles are not simply passive carriers; they are dynamic systems that can protect delicate molecules, cross biological barriers, and release drugs in a controlled way. I aim to utilise this technology to address two closely linked yet distinct diseases simultaneously: lung cancer and pulmonary fibrosis. On the surface, these conditions seem very different — one marked by uncontrolled cancer cell growth, the other by the build-up of scar tissue. At the molecular level, however, they share several features, including abnormal growth factor signalling, chronic inflammation, and changes in the extracellular matrix. This overlap makes their co-occurrence particularly destructive, as it complicates treatment and reduces the effectiveness of conventional therapies.
Current treatment approaches usually address each disease separately, often involving multiple drugs, different delivery routes, and complex dosing schedules. This can reduce effectiveness and is often associated with serious systemic toxicity. Nanotechnology offers a different way forward. By creating lipid nanoparticles that carry both paclitaxel, a widely used chemotherapy drug, and nintedanib, an anti-fibrotic agent, I aim to design a unified treatment strategy. These nanoparticles can be tuned to have the appropriate size and surface charge that facilitates their uptake by cells, a stable lipid composition, and release profiles that allow each drug to act over a longer period. Encapsulation also helps to limit the toxic side effects of the free drugs, while preserving their therapeutic benefits.
In the lab, I study these dual-drug nanoparticles in both monoculture and co-culture systems. Cancer cell lines let me examine proliferation and apoptosis after treatment, while lung fibroblasts provide a model for fibrotic behaviour. The co-culture system is especially important because it reflects, at least in part, the real interactions between tumour cells and fibroblasts. Once activated, fibroblasts create a stiffened tissue environment that also supports tumour growth. My hypothesis is that nanoparticles simultaneously delivering paclitaxel and nintedanib will not only inhibit cancer growth but also suppress fibroblast activation, thus disrupting this harmful synergy.
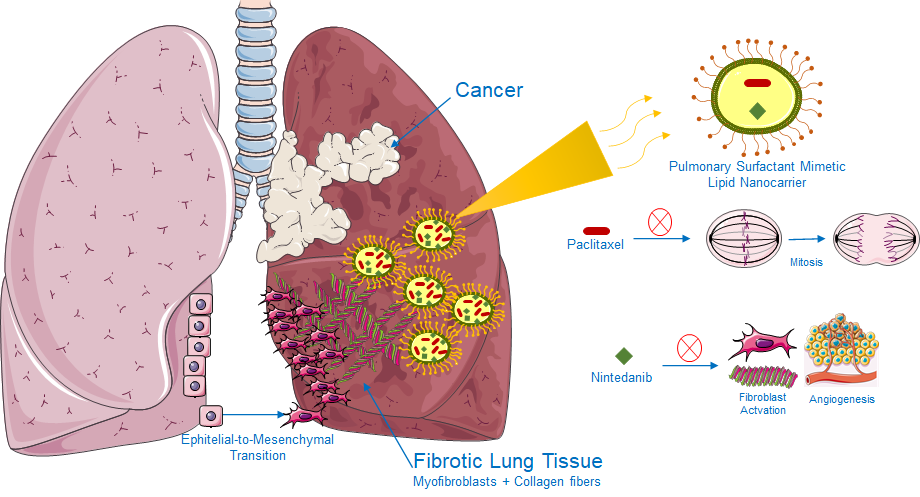
Figure1: Image adapted from Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
What motivates me most about this project is its translational potential. If successful, it could inspire the approach to complex lung diseases, offering patients a treatment that is both more effective and less distressing than current regimens. For those facing the dual diagnosis of lung cancer and fibrosis, such a therapy could mean improved survival and enhanced quality of life.
I also value being part of the NanoBio4Can network, which brings together early-career researchers across Europe with a shared commitment to advancing nanomedicine against cancer. The collaborative environment encourages exchange of ideas and constructive feedback, and it is rewarding to feel part of a collective effort. As my research progresses, I look forward to sharing both challenges and achievements, and to contributing to the broader vision of making nanotechnology a practical tool in oncology and respiratory medicine.
Author: Melike Belenli Gümüş, PhD
NanoBio4Can MSCA Co-Fund Fellow
TÜBİTAK MAM